In the collection of the Peabody Museum at Harvard University reside the mummified remains of a very peculiar creature. It has the shrunken head, torso and arms of a monkey, but from the waist down, it is a fish. This bizarre hybrid was bought by Moses Kimball, founder of the Boston Museum, from the family of a sea captain. Kimball leased it in 1842 to the impresario P. T. Barnum for his popular American Museum in New York City. Barnum claimed it was a mermaid found in Fiji.
In fact, such artifacts, typically intended for sale, were made from animal parts by fishermen and artisans in Japan at the time (although some of the mermaid seems to be fashioned from papier-mâché). Mythical hybrid beasts such as mermaids, centaurs and chimeras testify to our enduring fascination with the plasticity of biological form: the idea that natural organisms can mutate or be reconfigured. Both in legends and in fiction, from H. G. Wells’s 1896 novel The Island of Doctor Moreau to the 2009 movie Splice, we seem inclined to imagine living organisms as assemblies of parts that can be shuffled and rearranged at will.
But a crude stitching of components will not produce a viable organism. Bodies aren’t a collection of arbitrary pieces; a human embryo grows into a being with the standard features of a human body, all the parts working in synchrony. Biological forms seem to have inevitable, unique target structures.
An emerging discipline called synthetic morphology is now questioning that notion. It asks how, and how far, the natural shapes and compositions of living matter can be altered. The goal is not to create grotesque creatures such as the Fiji Mermaid but to understand more about the rules of natural morphogenesis (the development of biological form) and to make useful structures and devices by engineering living tissue for applications in medicine, robotics, and beyond.
Synthetic morphology might be considered the next stage of synthetic biology. The latter discipline has racked up impressive achievements in retooling cells for nonnatural tasks—for example, programming bacteria to glow in the presence of pollutants and other chemicals. Much of synthetic biology involves genetic engineering to introduce networks of genes that give cells new functions, such as manufacturing enzymes to make a nonnatural molecule.
Synthetic morphology works at the next level: controlling the shapes and forms into which many cells will assemble. Using the cells of multicellular organisms (like us), the technology might allow scientists to design entirely new tissues, organs, bodies and even organisms by exploiting the tremendous versatility and plasticity of form and function that seem to reside in living matter. The possibilities are limited only by our imagination, says bioengineer Roger D. Kamm of the Massachusetts Institute of Technology. We might design a novel organ, for instance, that secretes a particular biomolecule to treat a disease, similar to the way the pancreas secretes insulin. It could have sensor cells that monitor markers of the disease in the bloodstream, akin to controlled-release implants already used to administer drugs—but alive. Or, Kamm says, we could make “superorgans” such as eyes able to register ultraviolet light outside the visible spectrum.
Ultimately we can imagine creating entirely new living beings—ones shaped not by evolution but by our own designs. “By studying natural organisms, we are just exploring a tiny corner of the option space of all possible beings,” says biologist Michael Levin of Tufts University. “Now we have the opportunity to really explore this space.” Synthetic morphology poses deep questions that challenge the status quo in biology: Where does form come from? What rules has evolution developed for controlling it? And what happens when we bypass them? Doing so could turn on their heads our traditional notions of body, self and species—even of life itself.
The Rules of Living Form
Thinking of living matter as a substance that can be shaped and engineered at will was a revolutionary idea that arose in the 19th century. Zoologists had long regarded biological forms as innate, and Charles Darwin argued that natural selection sculpts them to be adapted to their environment. In the mid-1800s others, such as Darwin’s supporter Thomas Henry Huxley, began to suspect that there was a generic form of “living matter”—often called protoplasm—from which the most primitive life-forms were fashioned.
In his 1912 book The Mechanistic Conception of Life, German physiologist Jacques Loeb argued that life could and should be understood according to engineering principles. After discovering that he could stimulate asexual reproduction by treating unfertilized sea urchin eggs with simple salt solutions, he became convinced that nature’s way of doing things with living matter is not the only way. “The idea is now hovering before me,” he wrote, “that man himself can act as a creator, even in living nature, forming it eventually according to his will.”
Around the same time Loeb’s book was published, French physician Alexis Carrel developed techniques for growing tissues in a culture medium: a kind of unformed living material. He hoped it might become possible not just to preserve but to grow organs outside the body for transplantation when the natural ones wear out, thereby conveying the prospect of immortality.
That hasn’t happened, but tissue culture is now a well-established technology used to make, for instance, synthetic skin for grafts. It is now routine to cultivate living cells, including those of human tissues, in a petri dish, sustaining them with the nutrients they need to metabolize, replicate and thrive—much as we can grow colonies of bacteria or yeast.
The idea of cells as the “building blocks” of our bodies might make them seem rather passive, like mere bricks to be stacked in the masonry of tissues. But they are much smarter than that. Each cell is in many respects a living entity in its own right, able to reproduce, make decisions, and respond and adapt to its environment. Multicellular living matter concocts its own schemes, which means cells won’t necessarily stay in the same place or state.
This is strikingly apparent in the development of a new organism—a human being, say—from a single fertilized egg, or zygote. As that single cell becomes two, four and eventually many billions, it changes from what looks like an unstructured ball of identical cells to a body with a well-defined shape containing distinct tissues in which cells carry out different roles—producing the electrically coordinated contractions of the heart, for example, or secreting the hormone insulin in the pancreas.
Scientists and natural philosophers have wondered for millennia where this body plan comes from. How does the featureless blob that is the early embryo know what to make and where to make it? The answer, according to biology textbooks, is that the plan is contained in the cells’ DNA, encoded by genes. But this notion quickly falls apart. Yes, all the zygote seems to get by way of instruction is a genome, but you will look there in vain for any blueprint for a heart or brain. The genes simply encode proteins or other molecules that can ramp their production up or down.
It’s better to think of the molecular networks of the cell as encoding certain behaviors and tendencies, from which morphology emerges when those impulses play out among many cells. To understand—and perhaps ultimately to control—the forms of multicellular structures, we need to figure out these behavioral rules.
Cells produce order and form by communicating with and responding to one another. Each is bounded by a membrane studded with molecules, generally proteins. These molecules are capable of receiving signals at the cell surface and converting them into messages within the cell’s internal networks, typically ending with the activation or suppression of specific genes.
There are three main modes of communication for these externally derived signals. One is chemical: a molecule arrives at the cell surface and binds to a protein receptor there, triggering some change in the receptor that initiates a signaling cascade in the cell’s interior.
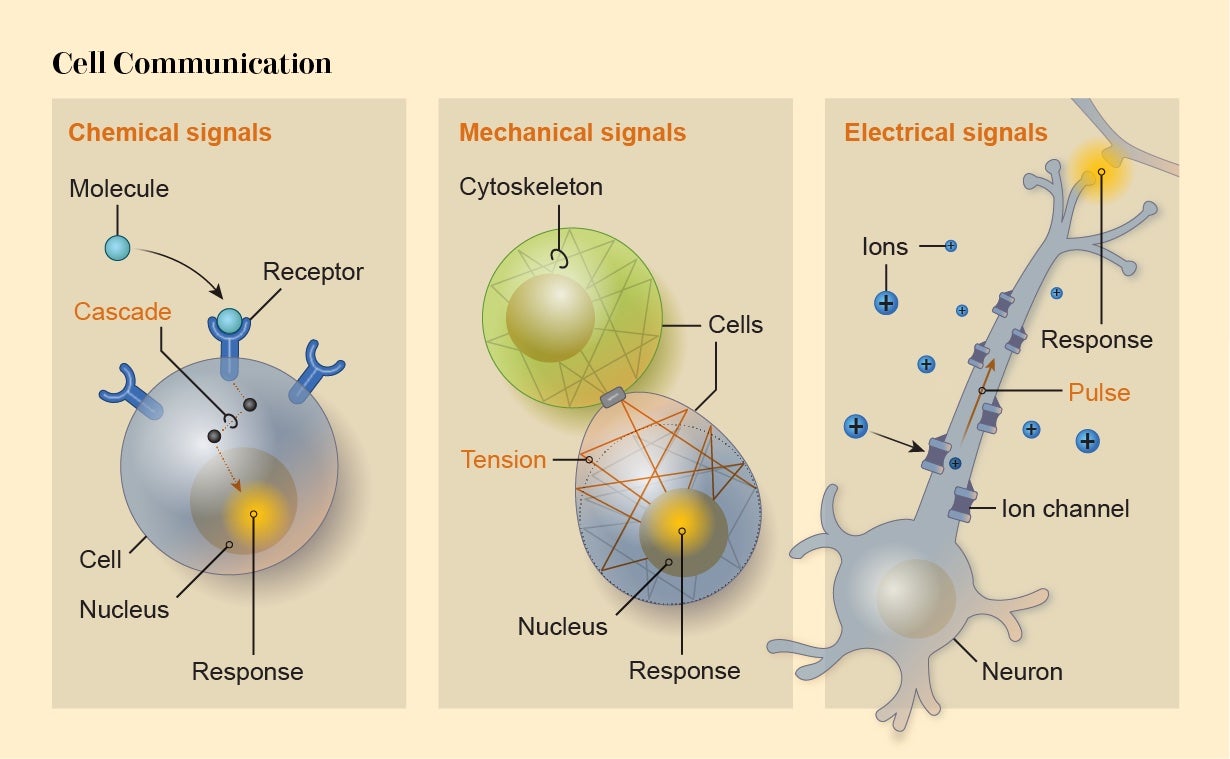
Alternatively, activity within a cell can be altered by mechanical signals such as stretching of the membrane when another cell sticks to and pulls it. Typically these mechanical signals are “transduced”—converted to some internal effect—by membrane proteins that alter their behavior when pulled or squeezed, to admit or exclude, for instance, electrically charged ions attempting to enter the cell.
The third mode is directly electrical. Ions passing across a cell’s membrane can make the cell electrically polarized. That’s how electrical signals are transmitted through heart muscle to induce regular contractions: the pulses travel from cell to cell via connections called gap junctions. Such electrical signaling is a capability shared by most cells.
Levin thinks bioelectric signaling between cells creates particularly powerful information-processing capabilities that can influence morphology. It therefore represents a useful “control knob” for applications in regenerative medicine and synthetic morphology, he says. Levin, Vaibhav Pai of Tufts and their colleagues have shown that the development of neural structures in the frog brain seems to be governed by the voltage across the membranes of embryonic cells. When the researchers permanently activated a key gene called Notch (one of the factors that induces precursor cells to become neurons in frog embryos), brain development was disrupted. But they were able to put it back on the right track by changing the membrane voltage of other cells nearby: the bioelectrical signal overrode the message coming from the genes, allowing proper morphogenesis to proceed.
Morphogenesis is a subtle process involving the interplay of information at the scales of the whole organism, the genetic and molecular activity in its cells, and everything in between—a complex mixture of bottom-up, top-down and middle-out signaling. If cells multiply faster in one part of the embryo than another, the developing tissue may buckle and fold. This deformation creates mechanical stresses that feed back into those cells to switch certain genes on and off, differentiating the cells from others around them and directing them along a developmental trajectory toward a particular tissue or organ.
In another example, as a mass of cells grows in a fetus, those in the interior might get cut off from the oxygen-ferrying blood pulsing down capillaries, triggering them to produce and release chemicals that induce some of their neighbors to develop into blood-vessel-forming cells. There was never any blueprint for a vascular system in the cells’ DNA; rather the eventual network of branching tubes is an emergent morphology produced by the rules of cell interaction and response.
“The genome specifies a cellular collective with massive plasticity,” Levin says, “which executes rearrangements until the correct target morphology is achieved.” One of the most striking illustrations of the existence of such target forms is the way a tube called the pronephric duct, a precursor to the kidney, grows in newts. If cells had genetic instructions telling them to assemble into a tube, we would expect bigger cells to make a proportionately bigger tube. In the 1940s, however, embryologist Gerhard Fankhauser tested this idea by using cells with extra chromosomes that made them grow larger than their usual size. He found that a tube of normal diameter and thickness developed—it just contained fewer cells. The largest cells changed shape to make the structure almost on their own. It was as if the cells collectively “knew” what their target structure was and adjusted their individual behavior accordingly. Albert Einstein was fascinated by these experiments, writing to Fankhauser that “what the real determinant of form and organization is seems quite obscure.”
An even more striking example of this apparent “overall vision” of multicellular structures is found in primitive flatworms called planarians. Cut a chunk out of a planarian, and it will regrow exactly those tissues that were removed, neither more nor less. Even a small part of a planarian can regenerate into a full worm with the typical shape and proportions. This capacity is all too evidently lacking in humans—so how do planarians do it? It seems to entail an ability of the regenerating cells to “read” the overall body plan: to take a peek at the whole, ask what’s missing and adapt accordingly to preserve morphological integrity. They are able to make use of top-down information. Levin believes this information is delivered to the cells via bioelectric signaling, which governs the maintenance of form in other organisms such as fish, frogs and humans. When he and his colleagues manipulated pieces of planarians to alter their bioelectric state, the regenerating cells produced unexpected anatomies—for example, worms with a head at each end.
Such regenerative potential is available to amphibians such as axolotls and salamanders, which can regrow limbs and tails that have been amputated. That feat demands two morphological capacities: the regrowing cells must be able to develop into many tissue types, such as skin, muscle, bone and blood vessel, and those tissues have to spontaneously organize themselves in the right way. Amphibians keep a reserve of such versatile cells, called stem cells, for repair jobs. If we are to find ways of imbuing our own bodies with regenerative powers, we need to know and master the global rules governing form.
The Plasticity of Cells
All embryos contain a ball of cells that are able to develop into any of the body’s tissue types, a property called pluripotency. In humans, however, these cells gradually lose this plasticity through a succession of transformations that differentiate them into specialized roles. It was long assumed that when these embryonic cells lose their pluripotency, that versatility is gone forever. But in 2006 biologist Shinya Yamanaka of the University of California, San Francisco, and his co-workers showed that this isn’t so. They were able to switch mature, differentiated mammalian cells back into a stem-cell-like state by injecting them with a cocktail of the genes that are active in embryonic stem cells (ESCs), essentially rewinding the clock of embryo development. Their experiment demonstrates that the fates of our cells, and the nature of our tissues and bodies, are far less inevitable and inexorable than people had thought: living matter is plastic and programmable.
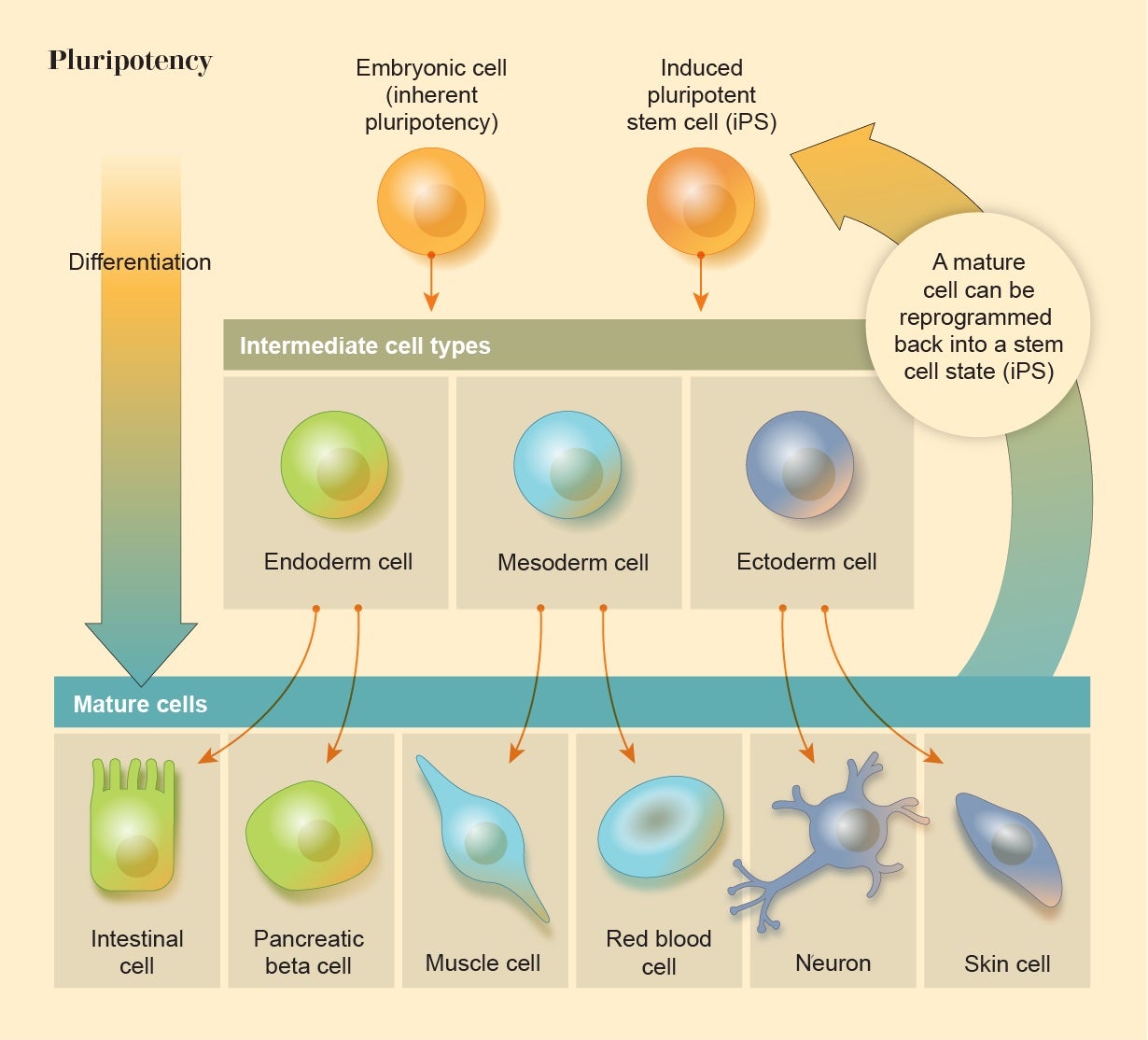
Cell reprogramming is now being explored for regenerative medicine. Some researchers are seeking to combat macular degeneration, a common cause of blindness, by reprogramming cells in the eye to support light-sensitive retinal cells. Others hope to cure neurodegenerative diseases such as Parkinson’s or spinal injuries by using neurons made from induced pluripotent stem cells (iPSCs) that can restore damaged connections in the nerve networks.
When cells are reprogrammed, they also acquire new morphological knowhow. For example, skin cells reprogrammed into iPSCs that are then cultured as neurons in a petri dish might not simply grow into a tangled mass. In the right growth medium, they might instead try to become a brain, recapitulating some of the structures seen in developing brains, with organized layers of cortexlike neurons and some of the characteristic folds seen in a mature cortex.
Such reprogrammed cells are not terribly good at making whole organs because they are missing some important information that, in an embryo, would come from the surrounding tissues. And currently such “organoids” can’t grow very large because they lack a vascular network, meaning the cells in the center eventually become starved of nutrients. To solve that problem, researchers are looking for ways to encourage some of the cells to develop into blood vessels. If transplanted into mice, liver organoids will spontaneously integrate with the animal’s own blood supply.
Another demonstration of the versatility of cells in multicellular structures is provided by so-called chimeric embryos, which contain cells from more than one type of organism. Because very different species usually can’t interbreed, monstrous hybrids such as the Chimera of Greek mythology seemed biologically implausible; the only way to make something like the Fiji Mermaid was to crudely stitch together lifeless carcasses. But at the level of individual cells, the species barrier isn’t as important as we might think. All cells speak much the same language, and those of different species seem to get along fairly well in an embryo. Scientists have created several chimeric animals—mosaics of cells of different species, such as the goat-sheep blend called a geep—by adding stem cells from one species to the embryo of another.
The further the evolutionary distance, the more precarious the chimera becomes. Some researchers are now experimenting to see whether “human” organs, made from human stem cells (either ESCs or iPSCs), can be grown in livestock animals such as pigs and cows to create a reservoir of organs for transplantation.
All this testifies to the fact that there is nothing fixed or inevitable about biological morphology at the level of cells. If that seems surprising, it is perhaps because we have been so wedded to the blueprint picture of developmental biology. But that picture demands excessive—in fact impossible—overspecification of the body plan. A blueprint could never, for example, dictate how every one of our 86 billion neurons should be wired up. All evolution needed to do was specify basic rules of cell communication and behavior that, when played out in the known, predictable environment of the womb or egg, would reliably create a specific morphology.
Perhaps that is the most efficient way to make complex organisms: not to program every cell to go to a particular place and become a specific thing in a paint-by-numbers fashion, but rather to give cells rules of interaction that enable them to figure the rest out for themselves. Change the environment, though, and those same rules might produce a very different end result. That was startlingly illustrated in recent work by Levin, Douglas Blackiston of Tufts and their colleagues. They simply broke up frog embryos into small pieces and left them to do what they would in a nutrient medium. “If we give them the opportunity to reenvision multicellularity,” Levin says, then “what is it that they will build?”
Over a couple of days the cells clumped into little clusters that began behaving like multicellular microorganisms, sprouting cilia, hairlike protrusions that beat in a synchronized way to propel the clusters through the fluid. These structures, which the researchers called xenobots (in reference to the Latin name of the original organism, the African clawed frog Xenopus laevis), will re-form their shape if damaged, suggesting that there is some kind of “goal” to their morphology. It was as if the genetic instructions in these cells, combined with the laws of cell interaction they support, could give rise to a completely different kind of organism than the frogs that would develop in normal circumstances. “We have the opportunity to make creatures in 48 hours that have never existed before,” Levin says. Now he is imagining making organisms that are reconfigurable and “immortal” in that “when they die, the individual cells crawl off and make their life alone and maybe rejoin again later into something else.”
Morphological Engineering
Organoids, chimeras and xenobots all suggest that cells can make stable entities other than those Darwinian evolution supplies. We can select and generate target morphologies by design. “We can definitely force cells to create shapes that are not natural,” says cell biologist Marta Shahbazi Alonso of the University of Cambridge. Working out the rules governing synthetic morphology, however, is a much harder task than figuring out how to build with blocks that have specific assembly rules, such as LEGO bricks.
With cells, the blocks are themselves changed by the assembly process. “In a simple mechanical world, you would have pieces that interact with each other following a set of rules to build more complex structures,” Shahbazi Alonso says. But, she adds, the “beauty of development”—and also the complication—is that “the process of building a structure changes the very nature of the building blocks. Throughout development there is constant cross talk from processes that happen at different scales of biological organization.”
Synthetic morphology, then, demands a new view of engineering in which we assemble objects from their basic components not in a simple assembly-line manner according to a blueprint. We must exploit rules of interaction to enable a desired structure to emerge as if by collective agreement of the parts—by recognizing that those parts themselves have a kind of agency. Computational biologist René Doursat of the Complex Systems Institute in Paris identifies four categories of processes involved in such morphological engineering: Agents can attach to one another in a programmed construction or assemble via swarmlike coalescing. Alternatively, a structure may develop via growth and multiplication of the components, or it can generate itself by repeating an algorithm, like that which produces the fractal forms of plants.
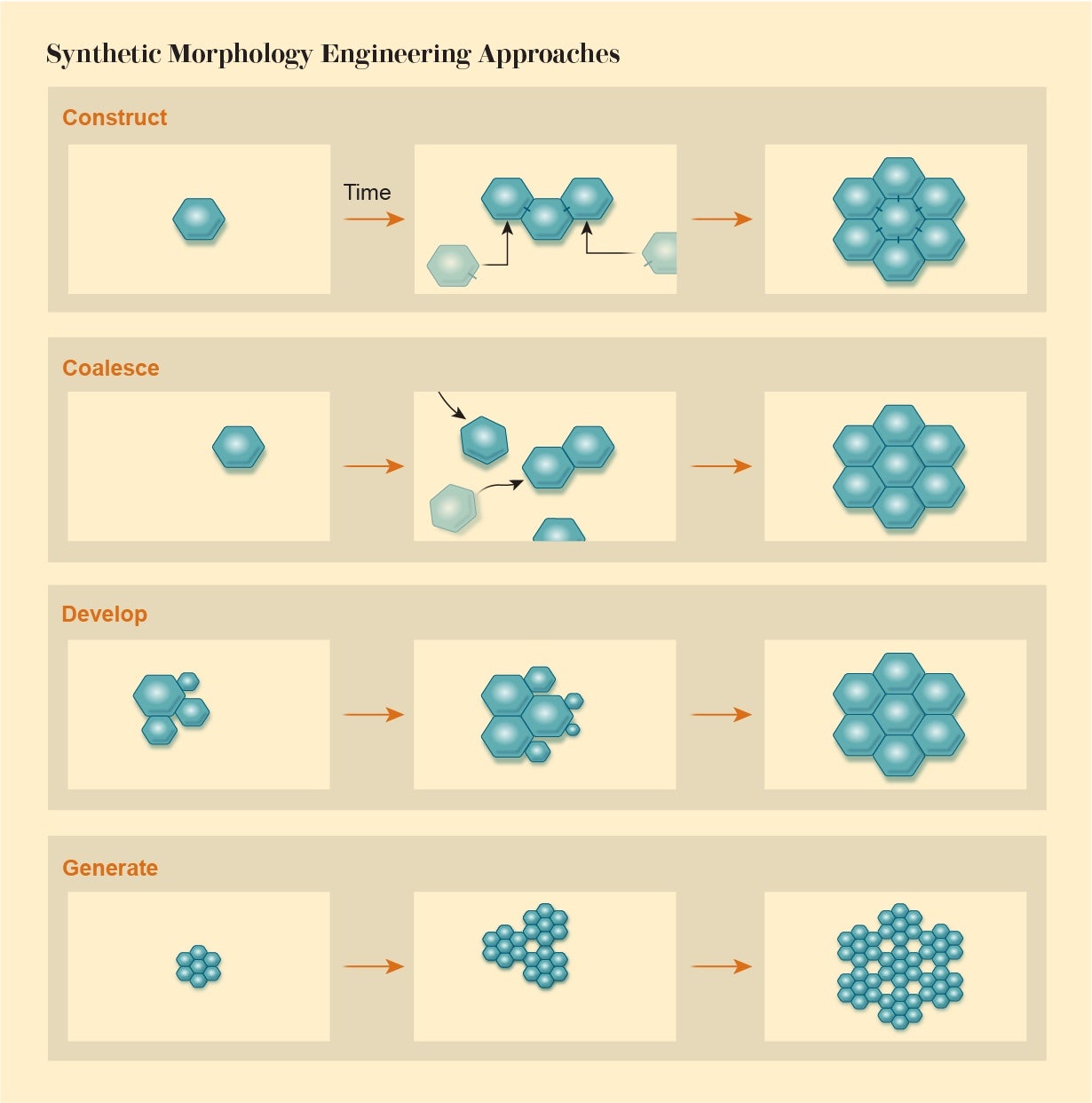
The challenge, Doursat says, is to find ways of ensuring reliable outcomes that will not be thwarted by small perturbations and that are adaptive—if circumstances change, the system needs to be able to find a solution that does the job. This philosophy has much in common with the way we create cities and societies: We have some idea of what we would like, but we can’t control it from the bottom up. We can only try to guide the self-organization along the right lines.
Doursat and his colleagues have proposed theoretical schemes for building with bacteria in this way, using synthetic genetic circuits to imbue them with interaction rules that will produce simple geometric elements made of many cells, such as rods and rings. Those shapes might then be assembled into higher-order structures. Some of the earliest work on multicellular synthetic biology also used bacteria. For example, Frances H. Arnold of Caltech, Ron Weiss of M.I.T. and their co-workers engineered a population of bacteria with genetic circuitry that allowed each cell to sense the population density in its environment and control the rate of spontaneous cell death, keeping the population growth within specified limits.
Kamm and Weiss, together with tissue engineer Linda G. Griffith, also at M.I.T., recently launched a multidisciplinary Center for Multi-Cellular Engineered Living Systems at the university, which builds on such work to create multicellular systems with specific functions by design. They believe that making these living systems will require a range of approaches, including everything from top-down patterning (where the cells are inserted into position “by hand”) to bottom-up self-organization (where the cells are programmed to self-assemble into a target structure).
Suppose you need to replace an artery and want to make a simple flow valve consisting of a blood-vessel-like tube of cells that is encircled at one point by a ring of muscle cells able to contract. You could make these two shapes out of a synthetic scaffold, such as a biodegradable polymer, and seed them with the two cell types, which would colonize the relevant components. That’s the top-down approach. Or you could start with a cluster of stem cells that can be tweaked and guided to differentiate in the right way while they move and coordinate with one another, eventually producing that same structure—that’s bottom-up, and more like the way the body builds such structures. The first approach may be simpler and could involve tools such as bioprinting, in which cells are delivered to specified locations by an inkjet-style device. But it might be harder to keep the resulting structure stable. What if different types of cells want to fuse or develop into other tissues? The bottom-up approach, in contrast, would build on stem cells’ ability to sustain themselves and make repairs if damaged.
Kamm says we don’t yet have good methods for reliably generating and predicting such outcomes. But they’re coming. One useful tool is optogenetics, which is already used to study the neural basis of behavior by switching specific neurons on and off. In this approach, scientists use genetic engineering to direct cells to make light-operated protein switches that control their electrical state. Fine laser beams can then be used to activate specific cells in a group and send them along particular developmental trajectories. Kamm says it might also be possible to selectively activate and differentiate cells mechanically (by poking them in various locations or using light-based optical tweezers to pull on them), thermally and bioelectrically (by, say, changing their membrane potentials at certain locations).
Building New Life
What should we build with such tools? One goal is to create living multicellular structures that resemble but don’t exactly mirror natural ones: a simplified, idealized tissue or organism, for instance, that helps to elucidate the processes that go on in the natural, more complex variety. Several researchers are assembling human stem cells into embryolike structures (“embryoids”) so they can watch the very early stages of embryogenesis in vitro.
If grown outside the uterus, the embryo cells don’t receive essential signals from their environment that would help orient and guide their development. They may begin to differentiate into the more specialized types that would eventually become part of tissues such as skin, blood and nerves—but it happens in a rather random, unstructured way. In 2014, however, Ali H. Brivanlou of the Rockefeller University and his co-workers showed that merely confining human ESCs within small circular “sticky” patches is enough to instill some order.
Brivanlou and other researchers are finding ways to make embryoids ever more like the real thing. Magdalena Żernicka-Goetz of the University of Cambridge and her colleagues have demonstrated that if they mix mouse ESCs with two other embryonic cell types (trophoblast stem cells and extraembryonic endoderm stem cells), they will organize themselves into a kind of hollow structure like a peanut shell that resembles the central amniotic cavity of real embryos. The cells seem to “know,” roughly, what an embryo looks like, and they not only organize themselves accordingly but also begin to differentiate into the correct specialized tissues.
It’s not clear how far these embryoids might be grown in vitro—but Żernicka-Goetz and others have made embryoids that will develop to the stage where limbs and organs start to form. If an embryoid were to be implanted in a womb—a procedure that would clearly be unethical in humans but might be contemplated in other animals—who knows what it might go on to do?
That’s not a rhetorical question. We can’t take it for granted that a synthetic embryoid will somehow find its way onto the usual track of embryo growth. It might pursue a different path entirely. That’s one reason for the lack of consensus on the ethical management of these entities. Should they be subject to the same rules and regulation that govern research with human embryos? Or are they a different thing entirely, one made of human cells on a different developmental path?
Robotic engineers are using living tissues as components in otherwise conventional robots. They generate behaviors that would be tricky to engineer with purely artificial materials and devices. Kit Parker of Harvard’s Wyss Institute for Biologically Inspired Engineering has collaborated with aeronautical engineer John Dabiri of Caltech and bioengineer Janna Nawroth of the Helmholtz Pioneer Campus in Germany to make a “medusoid,” a creature that looks like a jellyfish robot. It uses rat muscle tissue attached to a silicone polymer to produce undulating contractions, which allow it to swim like a real jellyfish. Parker and his colleagues also used rat heart muscle cells in a robot that swims by means of rippling motions modeled on those of the ray fish. By using optogenetics to control the activity of the muscle cells, the researchers were able to regulate the speed and turning motion of the robot so it could be guided by light through an obstacle course.
Chemical biologist Adam Cohen of Harvard and his co-workers, meanwhile, have made an “engineered bioelectric tissue” that can generate electrical oscillations. The electrically active cells in their structures were human embryonic kidney cells they engineered to produce ion-channel proteins, which let ions flow in or out to regulate the potential of the cell membrane. In some of the cells, the researchers used genetic engineering to add genes encoding other ion channels, enabling optogenetic switching with red and blue light. By combining these cell types in a ring, they made a light-activated structure that generated waves of electrical activity moving around the ring. The waves could be made to travel in either direction, meaning these structures could be used to encode binary information. Perhaps we could ultimately process data in a kind of living computer.
Understanding the rules that govern biological morphology might open up new possibilities for entirely artificial technologies such as robotics. James Sharpe of the European Molecular Biology Laboratory Barcelona and Sabine Hauert of the University of Bristol in England have programmed coin-sized cylindrical robots to self-assemble in swarms using principles that mimic those of living cells, communicating via short-range infrared signals. The swarms show a pseudobiological ability to form robust collective shapes that can adapt to damage and self-repair: a kind of inorganic, robotic tissue.
Levin thinks all this is just the start for synthetic morphology. “My conjecture is that cell collectives are universal constructors,” he says. Given a particular set of living components, we can make them do anything that is acceptable within the laws of physics.
But to do that, we’ll need a new mindset for engineering—one appropriate for dealing with materials that are not merely “smart” in the traditional sense of responding to their environment but that have genuine agency. This collaboration between engineers and their materials might entail letting go of some of our conventional categories for distinguishing machines, robots and organisms. Synthetic morphology implies that life can be remade if we relax the boundaries separating the natural from the artificial.







